
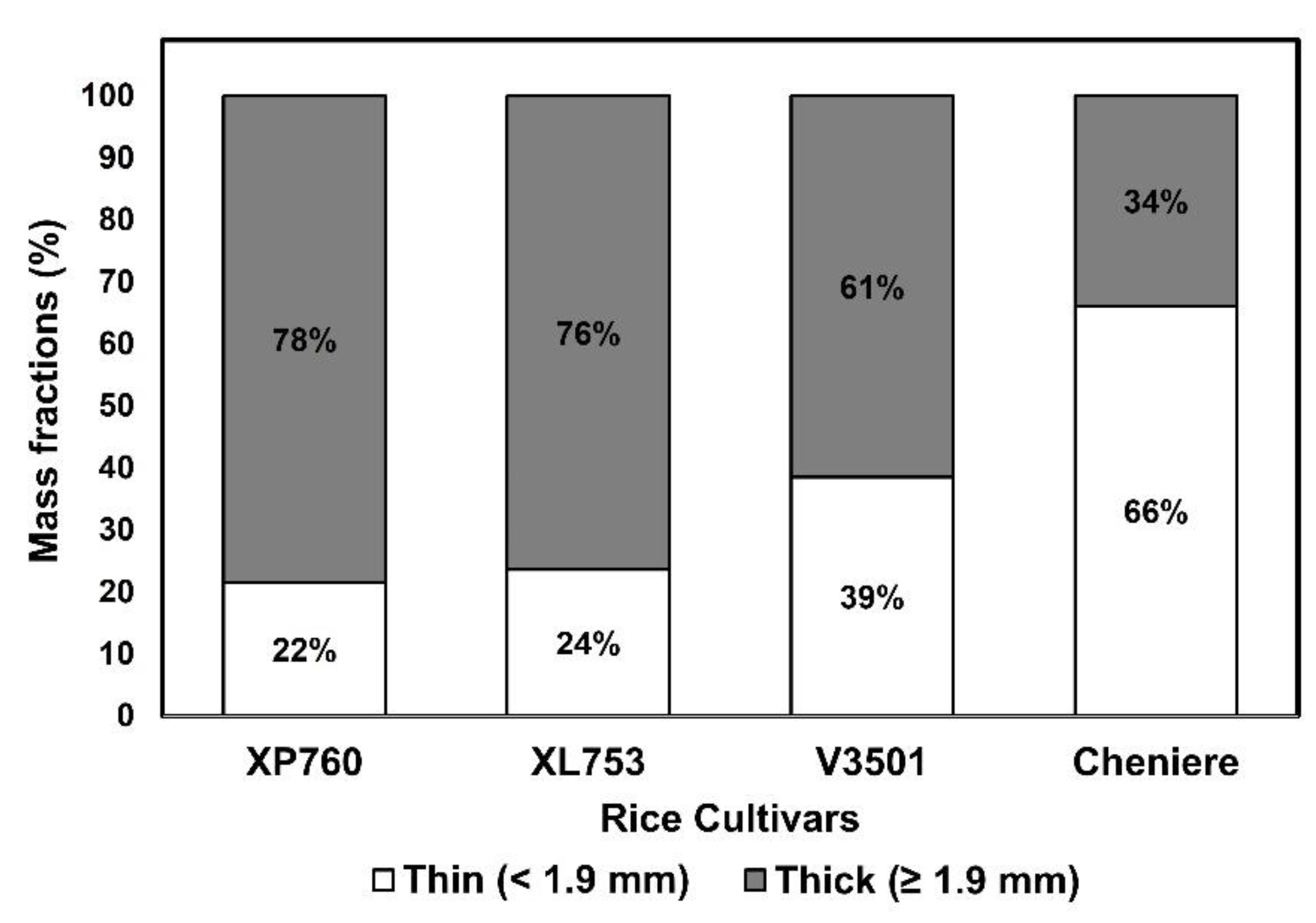
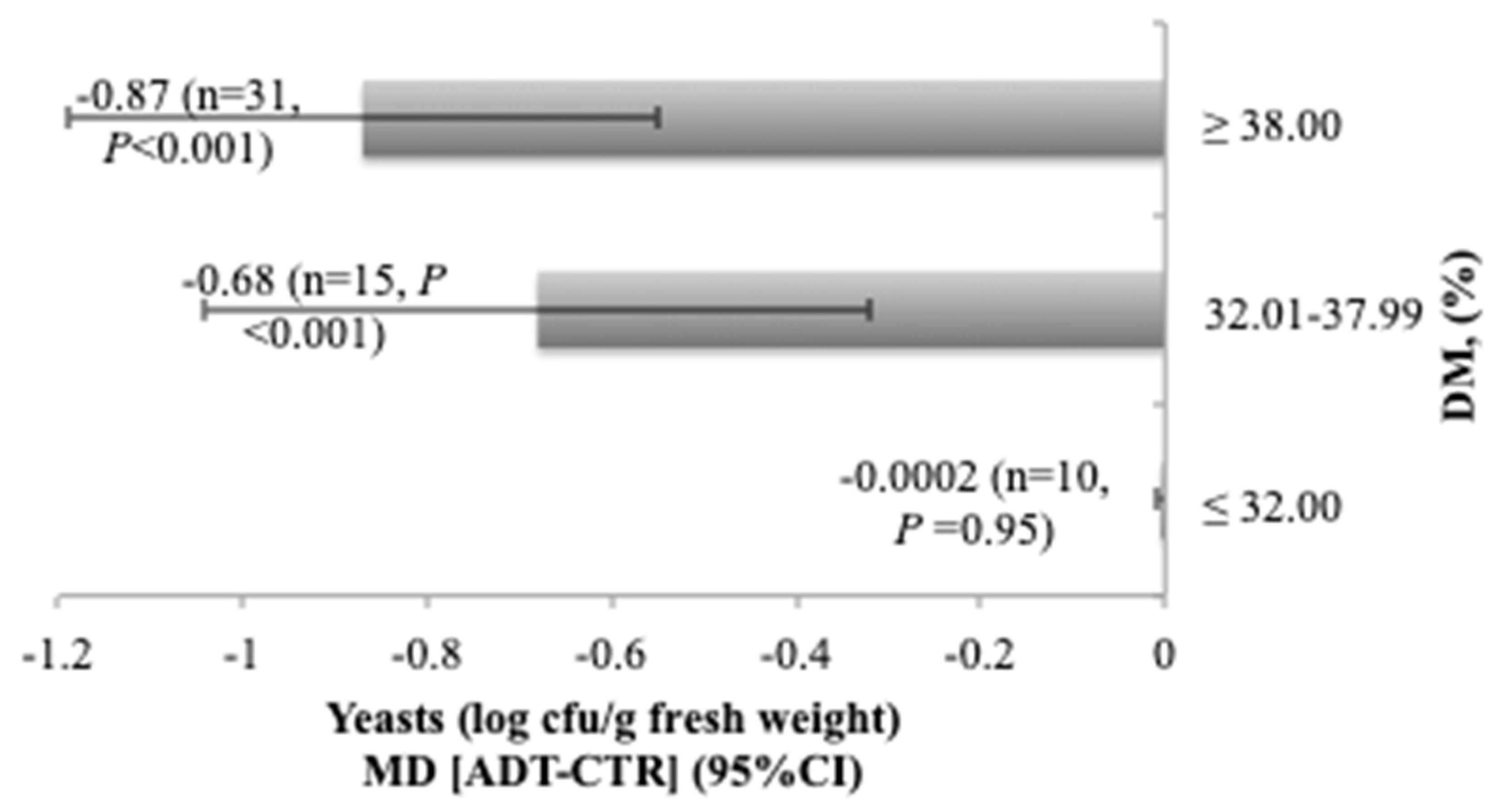
(1988) Calculating confidence intervals for some non-parametricĪnalyses. Statistical Methods inĪgreement between methods of measurement with multiple observations per
Measuring agreement in method comparison studies. The Lancet i:307-310.Ĭomparing methods of measurement: why plotting difference against Statistical method for assessing agreement between two methods ofĬlinical measurement. Introduction to medical statistics, 3rd ed. Matthews JNS (2002) Statistical methods in medical research. British Medical Journal 286:1489-1493.įor linear trends in proportions and frequencies. Gardner MJ, Pocock SJ (1983) Statistical guidelines for contributors to (1988) Calculating confidence intervals for regression and correlation. London: Chapman and Hall.Ĭonfidence intervals for the number needed to treat. Statistics and ethics in medical research. MS, PHD resident/ Director/ BiostatisticianĬopyrighted to the stated artist and web Designer. No strings attached! MathematicsĬopyright ? 2003 by SAS Institute Inc., Cary, NC, USA. Submit your site to dozens of top search engines for FREE. Submit your URL to a quality web directory Verizon Online: High Speed Internet Services Upper Limit for 2nd Stage Rejection of Drug (r)Įxpected Sample Size If Response Probability = P0 Upper Limit For 1st Stage Rejection of Drug (r1) Response Probability of Good Drug (P1) -> 0.60 Response Probability of Poor Drug (P0) -> 0.40 Wolf Grey 3s louis vuitton outlet wolf grey 3s jordan 6 sport blue sport blue 3s louis vuitton outlet louis vuitton outlet foamposites for sale sport blue 3s louis vuitton outlet louis vuitton uk louis vuitton outlet kate spade outlet Lebron 11 Wolf Grey 3s louis vuitton outlet coach outlet online sport blue 6s lebron 12 cheap jordansĮxample of this Calculation Your input Alpha -> 0.10 Beta -> 0.10 Oncology: Optimal Two-Stage Designs For Phase II Clinical TrialsĮnter probability of accepting poor drug (alpha) [between 0 andĮnter probability of rejecting good drug (beta) [between 0 andĮnter response probability of poor drug (P0) Įnter response probability of good drug (P1) [P1 should be Guidance for Non-Inferiority Clinical TrialsĦ. To be included in statistical section of the Clinical Study Report (CSR).ĥ. The purpose of this document is to provide guidelines on what information need Guidance for Contents of the Clinical Study Report Primary and important secondary study objectives.Ĥ. Purpose of this document is to provide guidelines for the creation of analysisĪnd associated documentation that are submitted to the FDA statistical reviewer Wolf Grey 3s louis vuitton outlet wolf grey 3s jordan 6 sport blue sport blue 3s louis vuitton outlet louis vuitton outlet foamposites for sale sport blue 3s louis vuitton outlet louis vuitton uk louis vuitton outlet kate spade outlet Lebron 11 Wolf Grey 3s louis vuitton outlet coach outlet online sport blue 6s lebron 12 cheap jordans The Optimal Two-Stage Designs For Phase II Clinical Trials Phase IIIĬlinical trials usually have a Data and Safety monitoring Board (DSMB), withīroad responsibility for monitoring the conduct of the trial.įor the Creation of Analysis Data Files and Documentation of Statistical
#SAS JMP INFLUENCE COOKS D TRIAL#
Study participants and the validity and integrity of the trial data. The oversight and necessity of interim monitoring to ensure the safety of the Trials are important, with particular emphasis on recentlyĭetermining the number of centers in clinical trials Statistical aspects of both the design and analysis of Performing the appropriate statistical analyses. Correct interpretation of the dataįrom such trials depends largely on adequate design and on Your successful FDA submissions, please get in touch with us.Ībout 10000 clinical trials are undertaken annually in allĪreas of medicine, from the treatment of acne to the To discuss more in detail how we can help you to
#SAS JMP INFLUENCE COOKS D SOFTWARE#
With the following statistical and mathematical software packages:īayesian, JMP, nQuery, MATHLAB, IBM-SPSS, NCSS/PASS. Studies to a successful FDA submission using CRT (Case Report Tabulation) forĮlectronic submissions, implementing SOPs and Working Guidelines. Knowledgeable of drug approval process, ICH We are familiar with FDA requirements and We have over 20 years of pharmaceuticalĮxperiences and clinical data manipulation. Therapeutic areas and our specialties are Oncology, Osteoporosis, Diabetes,Īlzheimer's disease and Cardiovascular, Chronic Obstructive Pulmonary Disease (COPD), devices such Elite SPY, surgical Or S-Plus/R.) for clinical trials in phases I-IV. Technical expertise in Statistical Analysis Plan, Reporting and interpretingĮfficacy tables, figures, listings using SAS In Statistics, Mathematics, and SAS programming


 0 kommentar(er)
0 kommentar(er)
